ELECTROPHYSIOLOGY AND IMAGING
From the essentials to state of the art techniques for neurophysiologists
The course offers a choice of intensive, hands-on practicals and students attend numerous demonstrations of high-level techniques through visits to labs at the Université Paris Cité, the Ecole Normale Supérieure, the Paris Brain Institute (ICM) and the Institut de la Vision.
BENCHES
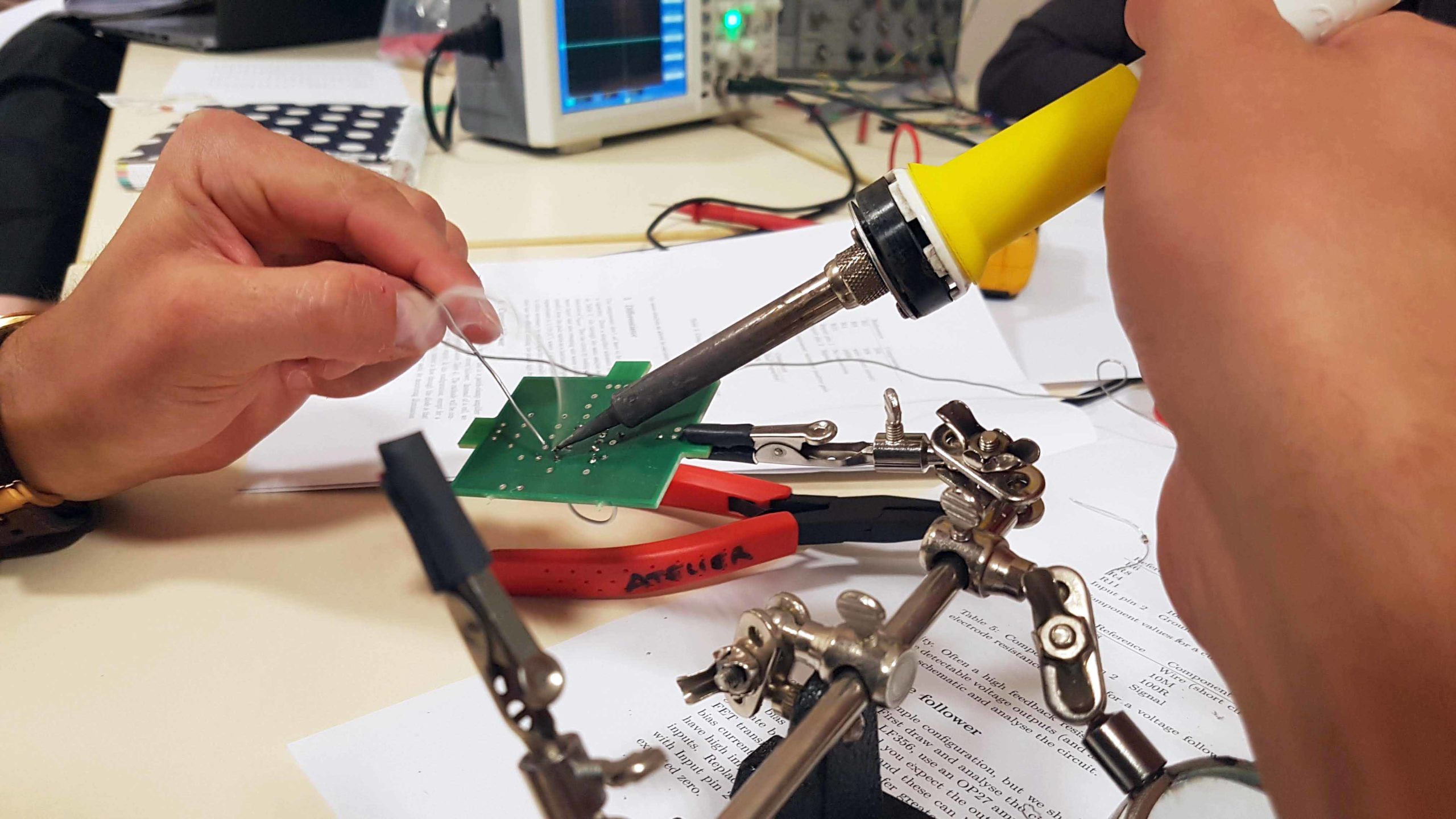
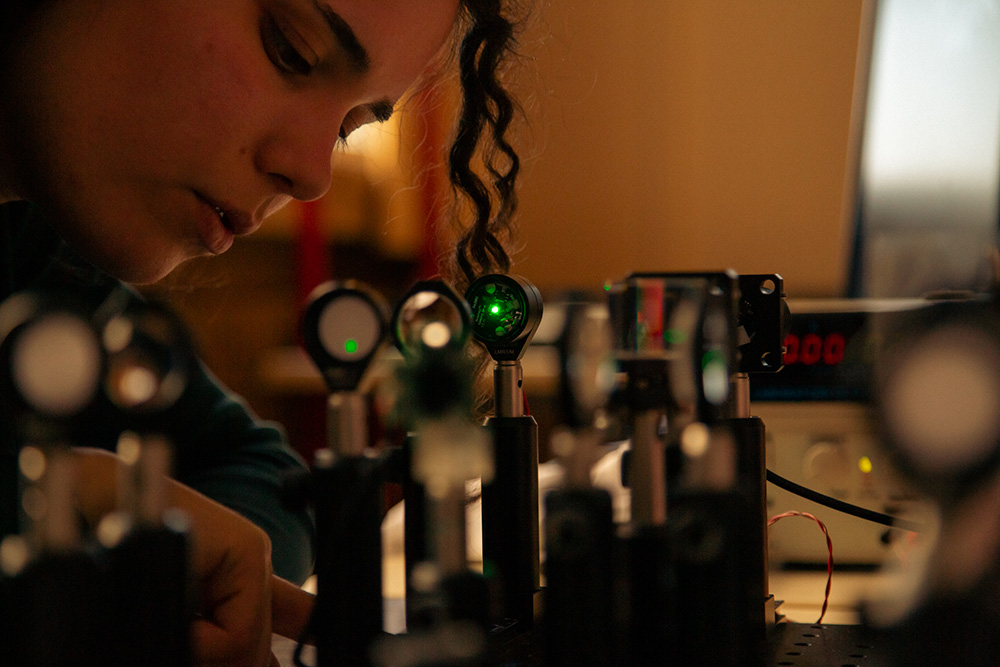
Consolidate your newfound theoretical knowledge of electronics and optics with the two practical “benches”. Everybody does these.
Intensive training on basic concepts
Electronics bench
Build operational-amplifier circuits illustrating the principles of electrophysiological recordings. Then use an Arduino to learn how digital electronics convert, represent and process information.
Optics bench
Reconstruct the image-forming path of a microscope and build a basic scanning system. Learn how to couple exetrnal optics to your own system.
HANDS-ON
Three two-day blocks, which students choose from the following :
Extracellular Field Recording
You will trigger and record field Excitatory Postsynaptic Potentials in hippocampal slices to study basic synaptic readouts, e.g. Paired-Pulse Ratio. You will use different protocols to induce Long Term Potentiation and test the effect of filtering on extracellular signals.
Advanced patching: cortex & hippocampus
Learn how to record from pyramidal cells and interneurones of hippocampus and cortex. Use extracellular stimulation to elict and record synaptic inputs. Record spontaneous inputs and probe firing responses. You will have access to a variety of modern equipment and a demonstration of loose-cell attached recording and stimulation.
Low-cost Open Source Miniscope
Learn how to assemble and deploy open-source miniscopes to perform in vivo calcium recordings in freely-moving animals.
Patch Clamp Training
If you want to learn patch-clamping or solidify your knowledge of the technique, this is the practical for you. We will explain the techniques, the preparations and how to optimise both current-clamp and voltage-clamp recordings. We will guide you through a step-by-step approach to locating the patch electrode, selecting a “good” cell, and approaching the membrane until achieving the famous giga-seal. You will also learn how to design recording protocols and choose the most intracellular solutions for your future experiments.
Acousto-optic 2-photon microscopy
You will learn about the powerful applications of 2-photon microscopy using acousto-optic deflectors: rapid random-access 3D scanning coupled with holographic excitation. These advanced techniques will be applied to imaging of genetically-encoded voltage and calcium indicators (GEVIs and GECIs), with part of the practical devoted learning about the latest developments in the fast-moving field of GEVIs, which can today be used to record both action potentials and subthreshold voltages from deeply located neurones in vivo.
High-speed imaging
Learn how to use ultra-fast CMOS camera imaging and to image neuronal signals in slices at the level of single cells or networks. The speed and flexibility of these cameras opens new avenues for exploring voltage, calcium and sodium dynamics of the most rapid neuronal mechanism of synaptic transmission and action potential generation.
Neuropixel electrodes
This two-day workshop provides hands-on training in Neuropixels electrophysiology. Day 1 covers data acquisition: building the probe and preparing it for acute recording, planning trajectories with 3D atlases, introduction to probe hardware (Neuropixels 1.0 vs 2.0, analog-to-digital conversion), denoising a rig, acute recording in awake mice, and signal synchronization. Day 2 focuses on data analysis: in-depth description of Neuropixels raw data format, introduction to machine learning relevant for spike-sorting, theory of spike sorting, practical data pre-processing, automated spike sorting and manual curation, and alignment of neural to behavioral data.
Subcellular 2-photon microscopy
You will be introduced to experimental strategies for investigating activity-dependent subcellular calcium and neurotransmitter signaling using 2-photon microscopy in vitro. Participants will gain practical experience with both organic and genetically encoded calcium indicators, as well as recently developed neurotransmitter sensors, including glutamate reporters. Strong emphasis is placed on experimental design, data acquisition, and quantitative analysis, equipping participants to rigorously interpret imaging data in complex neural systems.
Neuronal Network 2 photon
This hands-on workshop will take you inside the world of multiphoton microscopy of calcium signals, letting you explore brain tissue in remarkable detail while learning its principles and operation. Using brain slices, you will explore cellular and subcellular dynamics in real time. Observe microglia mobility and their response to brain injury, or monitor intracellular calcium fluctuations in neurons in response to electrical or pharmacological stimulation.
Advanced patching: cerebellum
Learn about cerebellar slices, how to patch and optimise recordings from Purkinje cells and granule cells. You will have the opportunity to use extracellular stimulation of synaptic inputs, such as parallel fibre and climbing fibre inputs to Purkinje cells. You will have access to a variety of modern equipment and a demonstration of loose-cell attached recording and stimulation.
Head-mounted 2P miniscope
During this practical, you will discover the fundamental principles and basic techniques of mini2P, a new type of head-mounted 2-photon miniscope that allows calcium recording in freely behaving animals. You will learn about the entire procedure, from surgery, through recording to data analysis with a big emphasis on a practical approach.
INSTRUCTORS
Tim Harris
Janelia Farm, USA
Luiza Filipis
Inserm Grenoble
Pierre Yger
Institut de la Vision
Brandon Stell
Université Paris Cité
Vincent Villette
IBENS
Yannick Goulam Houssen
IdA
Maxime Beau
UCL, UK
Marin Manuel
Univ. Rhode Island, USA
Desdemona Fricker
Université Paris Cité
Joana Lourenço
ICM
Nelson Rebola
ICM
German Sumbre
IBENS
Marco Canepari
Inserm Grenoble
Mahesh Karnani
VU, The Netherlands
Boris Barbour
IBENS
Eric Schwartz
Université Paris Cité
Benjamin Mathieu
IBENS
Ludovic Tricoire
IPS
Laila Blömer
Inserm Grenoble
Igor Delvendahl
University of Zurich
Michael Graupner
Université Paris Cité
Vincenzo Marra
University of Leicester
Nelson Rebola
ICM
Nicolas Gervasi
Collège de France

