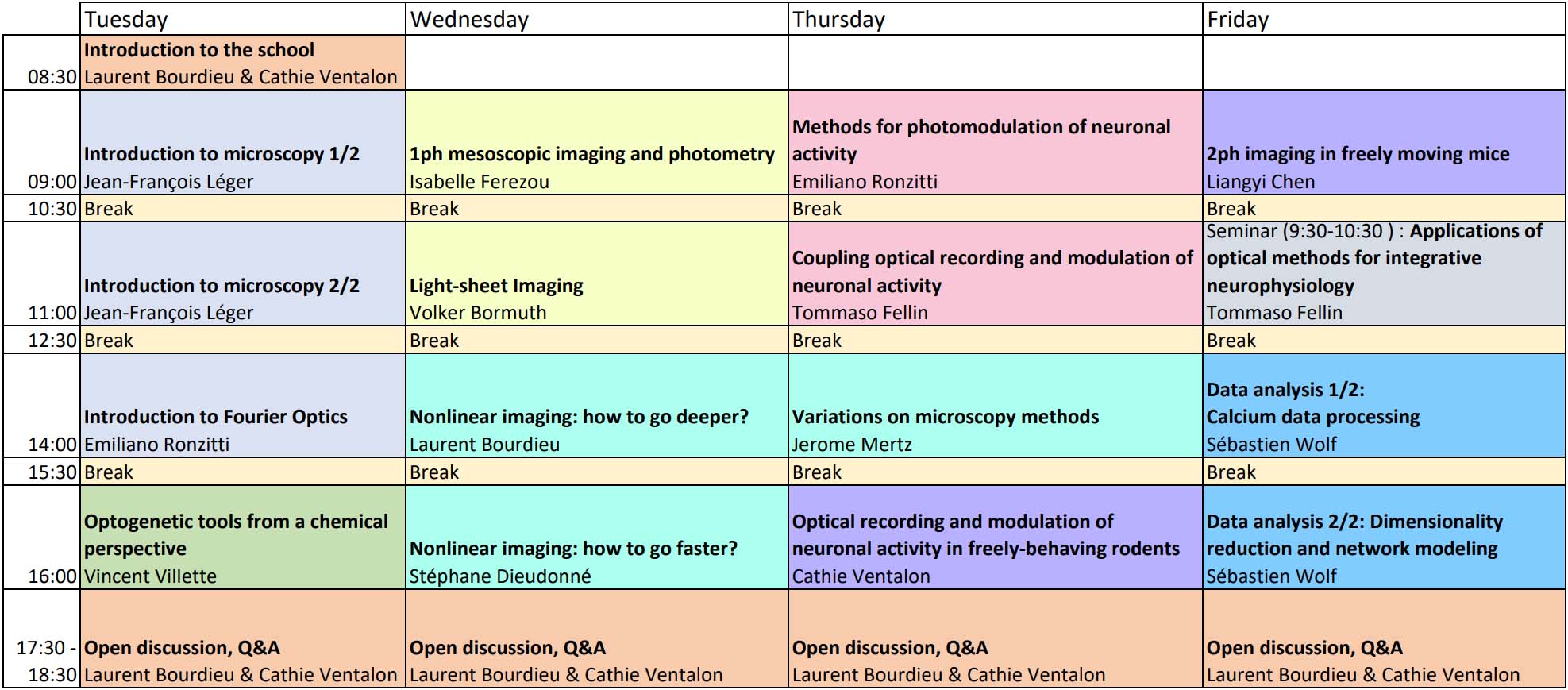
TIMETABLE
Times are French timezone
Subject to permission from the speakers, lectures will be recorded and made available to registered participants for the duration of the course and for a short time after it has concluded. This will allow you to catch up on any lectures you are unable to attend and also provide a backup in case of connection problems.
MONDAY – May 10th
15:30 Introduction – David Ogden
16:00 Basic optics
Noah Russell (MBA Plymouth, UK)
This introductory lecture will cover the basic physics of light and the use of lenses to both form an image of an object and to illuminate it. A Fourier-based description of microscopes will be given to help give a conceptual understanding of microscopy and its limitations. Finally a brief introduction will be given to concepts that will be covered in more detail later in the course including fluorescence imaging, light sources and detectors.
17:35 Optical principles and problems in microscopy
Brad Amos (LMB Cambrige, UK)
Brad Amos was originally trained as a zoologist, but has a lifetime interest in microscopy and optics. With John White, Richard Durbin and Michael Fordham, he helped to develop the optical system of the laser-scanning confocal microscope which was manufactured by Bio-Rad under license from the UK MRC.
Now retired, he works with the group of Gail McConnell in Strathclyde, Scotland, on mesoscopy and other innovative optical methods.

19:00 Paris Neuro social
Our famous rooftop cocktail
A
little
surprise
to virtually
gather around…
TUESDAY – May 11th
16:00 Light sources
Martin Thomas (Cairn Research)
We tend to think of microscopes as having a high numerical aperture, in that they typically collect light from a sample over as wide a range of angles as possible. This is often necessary to achieve good depth resolution, to maximise the light collection efficiency, or both. However, there is also usually a requirement for high magnification, which reduces the numerical aperture at the detector in proportion. The problem we address here is that the illumination pathway, being basically the reverse of the detection one, will also have a low numerical aperture, so the situation for the light source is rather like having to shine light through a tunnel. Both the theoretical and practical challenges of this requirement are discussed, together with a discussion of the various practical solutions.
Cairn Research Ltd is an independent optical instrumentation Company, founded by Martin in the dim and distant mists of the 1980s. Our primary activity is the design and sale of products for the detection and measurement of optical fluorescence. We are based on our own farm just outside Faversham in Kent, where Martin continues to design ever weirder and more wonderful products for the Company, of which the accompanying photo happens to show one such.. However in this activity he now has the support of many others, including our CEO Jeremy Graham who will be giving a talk on camera technology later in this course.

17:35 Light detectors
Klaus Suhling (Kings College London)
Detector development has played a significant role in advancing fluorescence microscopy.[1] I will describe the two key processes involved in converting light into electric signals: the photoelectric effect in photoelectronic vacuum detectors and electron-hole pair generation in solid state detectors. Both light detection methods have been used in single point detectors and cameras. I will describe how they work, discuss signal-to-noise considerations and what the future holds.
[1] L.M. Hirvonen and K. Suhling, Wide-field TCSPC: methods and applications Meas. Sci. Technol. 28 012003, 2017.
Klaus Suhling is a professor of physics at King’s College London. He trained as a physicist in the field of fluorescence spectroscopy. He develops and uses advanced multidimensional fluorescence imaging techniques such as Fluorescence Lifetime Imaging (FLIM) to understand the properties and interactions of macromolecules in the biological and biomedical sciences. He has extensively worked on photon counting imaging techniques and fluorescence spectroscopy and fluorescence imagin
WEDNESDAY – May 12th
16:00 Fluorescence lifetime imaging (FLIM)
Klaus Suhling (Kings College London)
Among the various fluorescence microscopy methods, fluorescence lifetime imaging (FLIM) has emerged as a key technique to image the environment and interaction of fluorophores in living cells.[1] The contrast in FLIM images is given by the fluorescence lifetime of the fluorophores, independent of their concentration.[2] I describe what fluorescence is, highlight the significance of the fluorescence lifetime, then give some examples of FLIM measurements and point to future developments.
[1] S. Ameer-Beg, K Suhling, M. Kuimova, Special issue on fluorescence lifetime imaging (FLIM): from fundamentals to applications, Methods Appl Fluoresc 8(4), 040401, 2020.
[2] K. Suhling, L.M. Hirvonen, J.A. Levitt, P.-H. Chung, C. Tregidgo, A. Le Marois, D. Rusakov, K. Zheng, S. Ameer-Beg, S. Poland, S. Coelho, R. Henderson and N. Krstajic. Fluorescence Lifetime Imaging (FLIM): basic concepts and some recent developments, Medical Photonics 27, 3-40, 2015.
17:35 Calcium indicators
Igor Delvendahl (University of Zurich, Switzerland)

THURSDAY – May 13th
16:00 Lasers for one- and two-photon microscopy and mesoscopy
Gail McConnell (University of Strathclyde, UK)
In the last 35 years, optical imaging has become one of the most important applications of the laser. This lecture will give an overview of how lasers work, description of key parameters of lasers relevant for biomedical imaging, considerations when choosing lasers for imaging studies, and some applications of lasers in one- and two-photon microscopy.
17:35 Scanning laser confocal and multi-beam microscopy
Stefanie Reichelt (Cancer Research, UK)
MONDAY – May 17th
16:00 Cameras for Dynamic Live Cell Imaging
Jeremy Graham (Cairn Research)
Since I last presented this talk in 2019, digital cameras for biological light microscopy have achieved near-perfection for most applications. Huge investment and innovation led by the mobile phone industry, and others, has produced sensors that have overtaken the resolution and field-of-view limits of the objective lens with close to 100% efficiency and high dynamic range. So what next?
In my talk I will present an overview of current scientific camera technologies and some thoughts on possible future developments…
As CEO of Cairn Research Ltd I am immersed in the interface between technologists and scientists, and privileged to learn from both, which, hopefully, helps enable our company to build effective solutions to novel and existing microscopy challenges. Historically we have been associated with physiological fluorescence imaging, but are now involved in numerous projects across the micro and macro bio-imaging worlds.
17:35 Genetically encoded calcium indicators (GECIs)
David DiGregorio (Pasteur Institute, France)
TUESDAY – May 18th
16:00 Viral expression strategies
Jonathan Bradley (IBENS, France)
17:35 Transgenic approaches to label neurons
Jean Livet (Institut de la Vision, France)
Approaches to express exogenous transgenes in neurons (e.g. fluorescent reporters, indicators, sensors and actuators) are conditioning many experiments in neuroscience. Understanding the advantages and drawbacks of these approaches is important when designing new experiments. This lecture will aim to provide an overview of currently available transgenic techniques as well as the different genetic tools to restrict gene expression in space and time, along with some practical advice on their use.
As the result of an online collaboration, participants of the Paris Spring School will be entitiled to attend (free of additional charge) the All-optical interrogation of neuronal networks in vivo course from May 25 to 28.
Click on the image to see the program
MONDAY – May 31st
16:00 Introduction to optogenetics
Guillaume Dugué (IBENS, France)
17:35 Photolysis
David Ogden (SPPIN, France)
TUESDAY – June 1st
16:00 Total Internal Reflection Fluorescence Microscopy TIRF
Martin Oheim (SPPIN, France)
17:35 Optical measurement of membrane potential
Marco Canepari (Inserm Grenoble, France)
WEDNESDAY – June 2nd
16:00 Super-resolution microscopy
Marc Guillon (SPPIN, France)
17:35 Imaging single molecules in live cells
Marianne Renner (IFM, France)
What can we learn from looking at individual molecules? Besides providing super-resolved images, single molecule imaging give access to an often-neglected aspect of living systems: molecular diffusion. The aim of this lecture is to give you the tools to understand single particle tracking experiments and to discuss about the importance of lateral diffusion of ion channels for neuronal function.
THUSDAY – June 3rd
16:00 Glutamate sensors
Katalin Török (St Georges's Univ. London, UK)
Visualising neurotransmission has been a long-time ambition of many an investigator. This lecture journeys through the story of the development of glutamate sensors highlighting recent breakthroughs and alluding to the burgeoning toolkit of further neurotransmitter and modulator sensors.
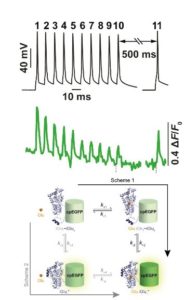
17:35 Tissue Clearing and Image Analysis
Nicolas Renier (Intitut du Cerveau, France)
MONDAY – June 7th
16:00 Miniscopes
Daniel Aharoni (UCLA, USA)
17:35 Whole-cell patch-clamp
Alain Marty (SPPIN, France)
TUESDAY – June 8th
16:00 Electronics primer
Boris Barbour (IBENS, France) / Marin Manuel (Univ. Rhode Island, USA)
We aim to distill with a maximum of intuition and a minimum of calculation the essential electronics knowledge that will allow you to understand electrophysiological recordings, focusing on effective analysis of operational amplifier circuits. The course content has been honed by years (decades…) of experience teaching at Plymouth and in Paris.
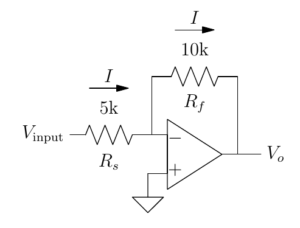
17:35 Electronics of patch-clamp amplifiers
Boris Barbour (IBENS, France) / Hubert Affolter (Sutter Instruments)
Modern patch-clamp amplifiers have many, many controls. Apart from killing cells, they actually have useful even critical functions. This lecture will allow you to understand how the controls should be used to obtain optimal recordings. Hubert Affolter designs control software for amplifiers at Sutter Instruments, Boris Barbour is a career electrophysiologist and has written this tutorial
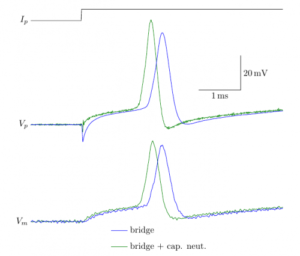
WEDNESDAY – June 9th
16:00 Functional roles of neuronal membrane conductances – dynamic clamp
Lyle Graham (Centre Giovanni Borelli CNRS, France)
17:35 Open Ephys
Josh Siegle, Pavel Kulik, Anjal Doshi (Allen Institute, USA)
Open Ephys is an initiative dedicated to the development and distribution of affordable yet powerful tools for neuroscience research. Open Ephys hardware and software is now being used to acquire extracellular electrophysiology data in hundreds of labs around the globe. This lecture will describe the equipment needed to build a high-channel-count ephys rig using open-source components, as well as a demonstration of the Open Ephys GUI (https://open-ephys.org/gui).
THUSDAY – June 10th
16:00 Extracellular recording
Clément Léna (IBENS, France)
17:35 Neuropixels – high-density extracellular recordings
Tim Harris (Janelia Farm, USA)
High capacity electrophysiology: How we got here and where can we go.
Modern microelectronics is transforming electrophysiology research tools and results. Most prominent in this space are the Neuropixels probes (now more than 6000 probes in 450+ labs) and INTAN multiplexer chips, the core of nearly every other recording system. This talk will review the history of ephys, show the origin of Neuropixels and other technologies, discuss the data digestion logjam this new capacity has generated, and discuss the various new probes (for primates and, even smaller probes from NIH funded projects) emerging from this effort.
![]()